Partnership Opportunities
 Research Track Program
Research Track Program SAS is one of the few research organizations with a dedicated research track program. Over the last decade, we have trained several researchers to become independent investigators. The program provides them with the tools, mentorship and opportunities through structured training and hands-on experience needed to excel in health research.
Program Highlights:
- Skill Development: Workshops and training sessions focused on advanced research methodologies, grant writing, and effective communication.
- Mentorship: Guidance from experienced researcher leaders.
- Career Advancement: Support for publishing in high-impact journals, presenting at conferences, and transitioning into leadership roles.
- Networking Opportunities: Access to a diverse community of peers, mentors, and collaborators, both locally and internationally.
We have successfully trained two cohorts of physician scientists under the Platform for Research Excellence Related to National Aims (PRERNA), Most of them have enrolled in or successfully completed their PhD from renowned universities. They have either been absorbed full-time in the organization or are now a part of the lead policymaking body for health research in India i.e., the Indian Council of Medical Research.
 Infant and Child Cognition Assessment Lab
Infant and Child Cognition Assessment Lab
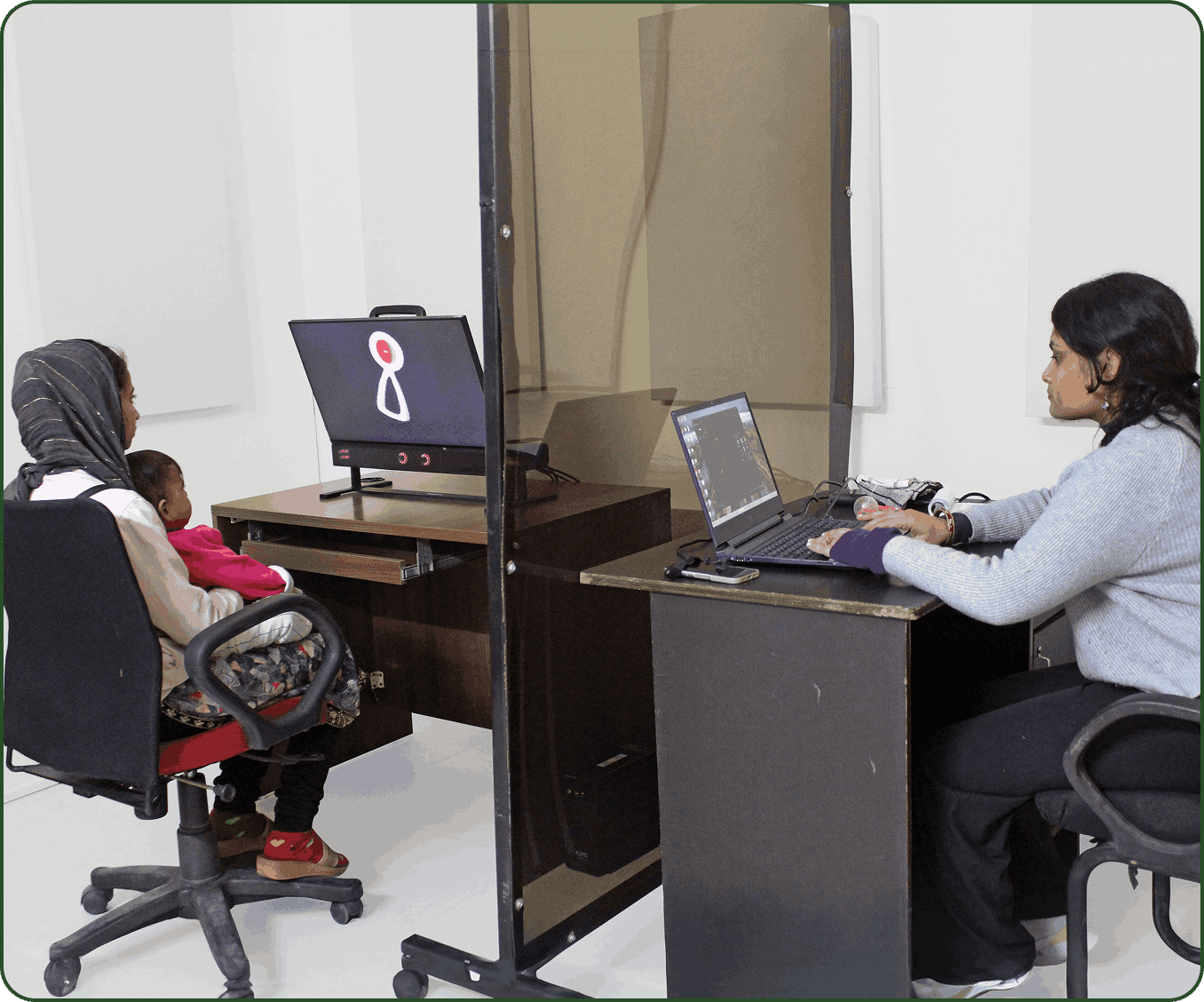
SAS is equipped with modern facilities designed to support advanced research on children’s brain development and early learning. One of its key tools is a screen-based eye tracker in the field office, which helps the researchers study how children focus their eyes, process information and achieve important early developmental milestones.
The lab also has specially designed cabins for working with children on developmental assessments. It offers a wide range of activities and tests tailored to measure a child’s growth in areas like thinking, problem-solving, language and school readiness. These include well-known tools like the Bayley’s Scale of Infant Development, the Wechsler Intelligence Scales for children of various ages, and the Ages and Stages Questionnaire, among others. These tools help researchers and professionals better understand how children are growing and learning, providing valuable insights into their cognitive, language and overall development.
 Body Composition Lab
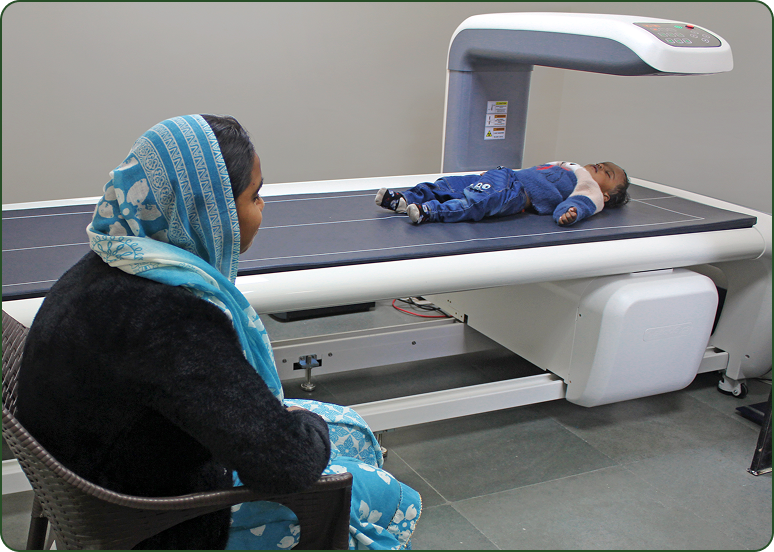
Body Composition Lab Building on our three decades of pioneering research in community nutrition, the Society for Applied Studies has made a significant advancement with the establishment of a Body Composition Lab.
DXA (Dual-Energy X-ray Absorptiometry) technology allows for a more comprehensive assessment of both the quantity and quality of growth. By integrating this robust technology, we can follow long-term cohorts with greater precision, linking growth patterns to future cardiovascular and metabolic health outcomes.
Our Body Composition Lab is set up in the field office and is registered with the Atomic Energy Regulatory Board. It is equipped with a state-of-the-art DXA device operated by trained staff. Utilizing narrow fan beam technology, the system ensures high-resolution imaging with ≤1.0% C.V. reproducibility for greater accuracy and reliability.
 Evaluation of Vaccines
Evaluation of Vaccines 
The vaccine clinical trial unit at SAS conducts regulatory trials for evaluating several nationally important candidate vaccines. The team has successfully conducted and continues to be actively involved in many phase II-IV studies for evaluation of vaccines for pediatric and adult populations including rotavirus vaccines, pneumococcal conjugate vaccine, combination routine childhood vaccines, HPV vaccine and vaccines against dengue, chikungunya, yellow fever, typhoid, paratyphoid and COVID-19. They are also engaged in evaluation of new devices and health products.
The team participated in all phases (I–IV) of the ROTAVAC® vaccine trials as well as the post-introduction evaluation.
SAS has a dedicated, fully equipped and functional Clinical Research Centre, established at Hamdard Institute of Medical Sciences and Research (HIMSR), for conducting vaccine trials. The team consists of skilled investigators and project managers with over 15 years’ experience and expertise in conducting trials in compliance with the national regulatory requirements and national and international ethical and good clinical practice guidelines. Several candidate vaccines tested by this unit have been granted licensure and are part of the national immunization program viz. ROTAVAC®, ROTASIIL®, PNEUMOSIIL®, MenFive®, HEXASIIL® and various COVID-19 vaccines.
 DSS (and GIS)
DSS (and GIS) SAS has set up a system called the Dakshin Delhi Environmental and Epidemiologic Surveillance System for Health and Disease (DEESHA) to study health and diseases in the urban areas of Dakshinpuri, South Delhi. This system tracks the health and well-being of the community, focusing on common illnesses and supporting research to improve vaccines.
The facility also includes a state-of-the-art Geographic Information System (GIS) lab, which helps create detailed maps and store location-based data. Using advanced tools and high-resolution satellite data, the GIS lab allows researchers to map and analyze health and living conditions in the community. This integration of mapping and health data provides a clearer picture of how health and social factors are connected, helping guide better public health decisions.

 Data Management
Data Management 
The center uses advanced technology to manage research data in real-time, using tablets and mobile phones connected to a secure cloud-based system. This allows for quick and accurate tracking of patient care and treatment follow-ups through a web-based platform. All processes follow strict regulatory standards to ensure quality and safety.
The center is well-equipped to handle its own research data and has extensive experience managing data for large, multi-site studies. It also has specialized systems to ensure the security of its data, including fire safety measures and restricted access to server rooms, all designed to meet international standards.
 Clinical and Research Laboratories (CRL)
Clinical and Research Laboratories (CRL) The in-house laboratory at SAS is specially designed to meet the highest standards for supporting research and medical care for study participants. It follows strict international and national guidelines for safety and quality, ensuring accurate and reliable results. The lab is equipped to handle and store samples, with advanced systems to process and preserve them, including deep freezers, automated analyzers for blood and biochemical tests, and specialized machines for safe and precise lab work.
The lab also prioritizes safety and environmental responsibility. The Institutional Bio-Safety Committee ensures that all activities meet strict health and safety standards, including proper waste management, emergency preparedness, and safe handling of chemicals and equipment. Regular audits confirm the lab’s compliance with design and safety protocols.
For managing its vast collection of samples, the lab uses a cutting-edge tool for tracking and organizing samples electronically. This ensures that all data is secure, accessible and well-maintained, supporting high-quality research and participant care.

 Biorepository
Biorepository 
Over the years, SAS has built a valuable collection of biological samples, including blood serum from mothers and infants, breast milk from mothers, and saliva from mother-infant pairs, collected at different times. These samples are carefully stored in liquid nitrogen tanks and ultra-cold freezers at -80°C, ensuring they remain in perfect condition under strict temperature-controlled conditions.
These are used for research purposes to enhance our understand of the factors affecting growth and development in early life.
 Setting up Mother Neonatal Care Units (MNCUs) and Kangaroo Mother Care (KMC) Wards
Setting up Mother Neonatal Care Units (MNCUs) and Kangaroo Mother Care (KMC) Wards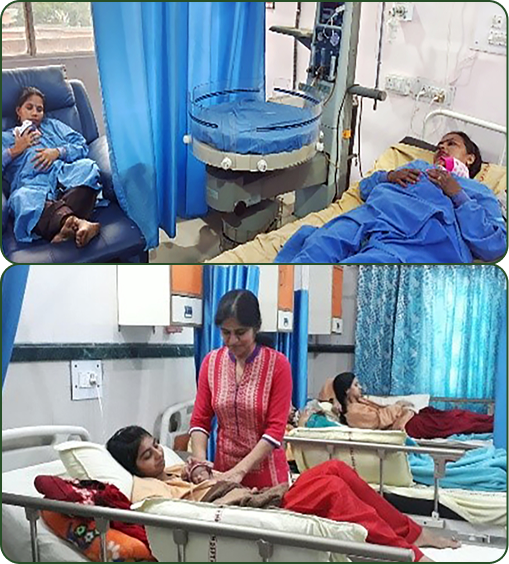
With over three decades of field-tested experience, the team has an excellent track record of establishing KMC wards in 16 district hospitals in Haryana, several hospitals in Delhi, Himachal Pradesh and Pune, in collaboration with the state governments, to promote KMC in stable preterm and/or LBW babies. Established in 2018, these wards have thrived, evolving into a durable cornerstone of newborn care. Seven years on, their continued success is a testament to unwavering, well-resourced government stewardship that has transformed an early implementation research project, into a long-term, high-impact standard for hospitals across the system.


